ethyne lewis structure|C2H2 (Acetylene : Pilipinas To be the center atom, ability of having greater valanceand being a electropositive element are important facts. however, from our experience we know that there is a very low possibility that hydrogen being a center atom in a molecule. 1. It is straight forward . Tingnan ang higit pa Albie Casiño is truly enjoying his dad era! On Instagram, Albie’s girlfriend Michelina shared some snaps from their family day in the coastal town of Bar Harbor in Maine. In the photos, Albie .
ethyne lewis structure,C2H2 (acetylene or ethyne) contains two carbon atoms and two hydrogen atoms. There is a triple bond between carbon atoms. There are no lone pairs on carbon or hydrogen atoms. In this tutorial, we are going to learn how to draw lewis structure of C2H2. Tingnan ang higit paThere are only two elements in C2H2; hydrogen and carbon. Hydrogen is a group IA element and has only one electron in . Tingnan ang higit pa
Total valance electronspairs = σ bonds + π bonds + lone pairs at valence shells Total electron pairs are determined by dividing . Tingnan ang higit pa
After deciding the center atom and basic sketch of C2H2molecule, we can start to mark lone pairs on atoms. Remember that, there are . Tingnan ang higit paTo be the center atom, ability of having greater valanceand being a electropositive element are important facts. however, from our experience we know that there is a very low possibility that hydrogen being a center atom in a molecule. 1. It is straight forward . Tingnan ang higit paLewis Structure for C2H2 (Ethyne) Commonly Tested Lewis Structures. We draw Lewis Structures to predict: -the shape of a molecule. -the reactivity of a molecule and how it .
Wayne Breslyn. 771K subscribers. Subscribed. 2.1K. 285K views 11 years ago. A step-by-step explanation of how to draw the C2H2 Lewis Dot Structure (Ethyne .Learn how to draw the Lewis structure for C2H2 (ethyne or acetylene), a hydrocarbon compound with two triple bonds. Find out the properties, hybridization, polarity, and . Wayne Breslyn. 771K subscribers. 197. 37K views 1 year ago. A step-by-step explanation of how to draw the C2H2 Lewis Dot Structure (Acetylene (Ethyne)). For the C2H2 structure use .
399K views 13 years ago. A step-by-step explanation of how to draw the C2H4 Lewis Dot Structure (Ethene). For the C2H4 structure use the periodic table to .C2H2 (Acetylene C2H2 is a chemical formula for Ethyne, a gaseous alkyne hydrocarbon. It has been used widely for welding and cutting purposes. This molecule is also known by the name Acetylene. The compound has . Alkynes are organic molecules made of the functional group carbon-carbon triple bonds and are written in the empirical formula of \ (C_nH_ { 2n-2 }\). They are unsaturated hydrocarbons. Like alkenes .Back. 70 More Lewis Dot Structures. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. The exception, of course, being the hydrogen's. .
Ethyne is built from hydrogen atoms (1s 1) and carbon atoms (1s 2 2s 2 2p x1 2p y1 ). The carbon atom does not have enough unpaired electrons to form four .
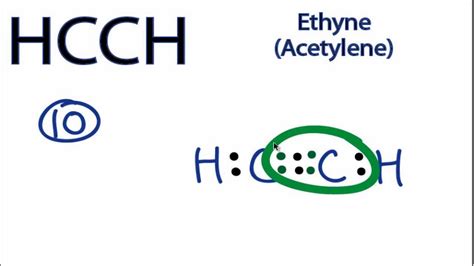
C 2 H 2 (acetylene or ethyne) contains two carbon atoms and two hydrogen atoms. There is a triple bond between carbon atoms and hydrogen atoms are joint with carbon atoms though sigma bonds. There are no lone pairs on carbon or hydrogen atoms. In this tutorial, we are going to learn how to draw the lewis structure of C 2 H 2 step by step.

A step-by-step explanation of how to draw the C2H2 Lewis Dot Structure (Acetylene (Ethyne)).For the C2H2 structure use the periodic table to find the total n.Lewis Dot of Ethyne (Acetylene) C 2 H 2. Back. 70 More Lewis Dot Structures. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. The exception, of course, being the hydrogen's. They follow the duet rule (2 electrons). Acetylene is an unsaturated hydrocarbon with a triple bond.Drawing the Lewis Structure for HCCH. HCCH (Ethyne) can also be written as C 2 H 2. Ethyne is also called Acetylene. For the HCCH Lewis structure you'll need to form a triple bond between the two carbon atoms. Hydrogen atoms only need two electrons for a full outer shell. There are a total of 10 valence electrons for the HCCH Lewis structure.
Drawing the Lewis Structure for C 2 H 2 - Ethyne or Acetylene. Viewing Notes: With C 2 H 2 you are going to run out of valence electrons and will have to share more than one pair of electrons between the Carbon atoms.; Remember that Hydrogen (H) atoms always go on the outside of a Lewis Structure. Note that Hydrogen only needs two valence electrons .A. Chemical formula of Ethyne. The chemical formula of ethyne is C2H2. It consists of two carbon atoms and two hydrogen atoms. Ethyne is a colorless gas with a distinct odor and is highly flammable. The C2H2 Lewis structure and its geometry help to understand the bonding, reactivity, and properties of the molecule. II.H. 4. ) Lewis Structure, Hybridization. Ethene's lewis structure can be built by VSEPR rule. Most stable structure is taken as the lewis structure of ethene. Hybridization of atoms in ethene molecue can be found from lewis structure. Each step of determining the lewis structure of ethene and hybridization are explained in this tutorial. A step-by-step explanation of how to draw the C2H4 Lewis Dot Structure (Ethene).For the C2H4 structure use the periodic table to find the total number of val.
C2H4 Lewis Structure, Molecular Structure, Hybridization, Bond Angle and Shape. The chemical formula C2H4 represents Ethylene. This molecule is also represented by H2C=CH2, clearly showing the alkene nature of the compound. An alkene is a hydrocarbon with a Carbon-Carbon double bond. C2H4 exists as a colorless gas and .
Ethene is the formal IUPAC name for H 2 C=CH 2, but it also goes by a common name: Ethylene. The name Ethylene is used because it is like an ethyl group ( CH2CH3 C H 2 C H 3) but there is a double bond between the two carbon atoms in it. Ethene has the formula C2H4 C 2 H 4 and is the simplest alkene because it has the .
Step 3: Connect each atoms by putting an electron pair between them. Now in the C2H2 molecule, you have to put the electron pairs between the carbon-carbon atoms and between the carbon .
The correct Lewis structure for ethene is shown below: In the molecule ethene, both carbon atoms will be sp2 hybridized and have one unpaired electron in a non-hybridized p orbital. These p-orbitals will undergo . Ethyne (HCCH), commonly known as acetylene, has a linear structure with a carbon-carbon triple bond. Each carbon (C) atom, with 4 valence electrons, is bonded . The Lewis structure shows two C atoms connected by a triple bond and each C atom bonded to an H atom. This arrangement uses 10 valence electrons: 2 for each C-H bond . Ethylene is a colorless gas with the chemical formula C2H4. It is a hydrocarbon with two carbon connected with a double bond. In this article, we will study ethene (C2H4) lewis structure, molecular geometry, .ethyne lewis structure C2H2 (Acetylene Hi Guys! Welcome to Geometry of Molecules, and today in this video we are going to help you know the step-by-step method for determining the Lewis Structure .

Ethylene (commonly knows as ethene), CH 2 CH 2, is the simplest molecule which contains a carbon carbon double bond. The Lewis structure of ethylene indicates that there are one carbon-carbon double bond and four carbon-hydrogen single bonds. Experimentally, the four carbon-hydrogen bonds in the ethylene molecule have been .Description. Sa structure est linéaire : . Il a été dit que Berthelot, en 1862, fut le premier à synthétiser l’acétylène, dans un appareil surnommé « œuf de Berthelot », par la production d'un arc voltaïque entre deux électrodes de graphite baignant dans une atmosphère d’hydrogène : . 2 C + H 2 → C 2 H 2.. Toutefois, le professeur Morren, doyen de la .ethyne lewis structure Ethene or C2H4 is a common straight-chain acyclic alkene and an important member of organic hydrocarbons. Having a double C=C bond, it is unsaturated and this gives rise to several properties. Here, we learned about how to draw the proper Lewis Structure and find out the molecular geometry of an ethylene molecule.
ethyne lewis structure|C2H2 (Acetylene
PH0 · Lewis Structure for C2H2 (Ethyne)
PH1 · Lewis Dot of Ethyne (Acetylene) C2H2
PH2 · How to Draw the Lewis Structure for C2H4
PH3 · How to Draw the Lewis Dot Structure for C2H2: Acetylene (Ethyne)
PH4 · C2H2 Lewis structure, Molecular Geometry, Hybridization & Bond angle
PH5 · C2H2 Lewis structure, Molecular Geometry,
PH6 · C2H2 Lewis Structure Tutorial
PH7 · C2H2 (Ethyne) Lewis structure
PH8 · C2H2 (Acetylene
PH9 · Bonding in Ethyne (Acetylene)
PH10 · 3.7: Alkynes